Compounds Can Be Split Apart:
The reverse of the element-combination procedure can take place with many compounds. Water is an outstanding illustration. Whenever water is electrolyzed, it divides into hydrogen and oxygen gases.
You can perform the following electrolysis experiment at home. Build the two electrodes out of large nails. Wrap few bell wires about each nail near the head. Add a cupful of ordinary table salt to a bucket full of water, and liquefy the salt thoroughly to make the water into a logically good electrical conductor. Fix the two electrodes to opposite poles of a 12-volt (12-V) battery made from two 6-V lantern batteries or eight ordinary dry cells connected in series. Insert the electrodes into the water a few centimeters distant. You will see bubbles rising up from both the electrodes. The bubbles coming from the negative electrode are hydrogen gas; the bubbles coming from the positive electrode are oxygen gas as shown in figure below. You possibly will see a lot more hydrogen bubbles than oxygen bubbles.
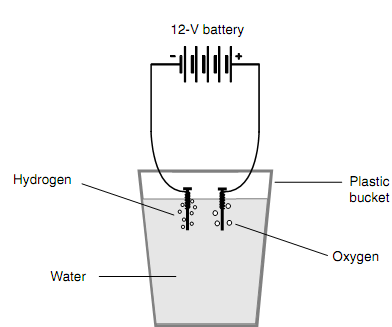
Figure: Electrolysis of water, in which the hydrogen and oxygen atoms are dividing apart from the compound.
Be cautious whenever doing this experiment. Do not reach into the bucket and capture the electrodes. Although, you should not grab the electrodes or the battery terminals at all. The 12 V supplied by the two lantern batteries is sufficient to give you a nasty shock whenever your hands are wet, and it can even be very dangerous.
When you leave the apparatus which is as shown in the figure below running for a while, you will start to notice corrosion on the exposed wire and the electrodes. This will particularly occur on the positive electrode, where oxygen is attracted. Recall that you have added table salt to the water; this will attract chlorine ions as well. Both chlorine and oxygen combine readily with the copper wire and the iron in nail. The resultant compounds are solids which will tend to coat the wire and the nail after a period of time. Eventually, this coating will act as an electrical insulator and decrease the current flowing through the saltwater solution.