Ternary structures
Ternary structures are ones with three elements exist, instances being CaCO3 and CaTiO3. Oxides are the most common instances of such type of structures and exemplify some of the significant principles. Two fundamentally distinct structural features are possible, as follows:
- Complex oxides are compounds consisting of complex ions, that appear as discrete structural units. For instance, calcium carbonate has a structure based on rocksalt with the distinct sites occupied by Ca2+ and CO2-3 ions.
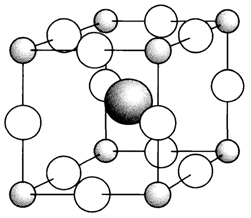
- Mixed oxides are exemplified by CaTiO3 that, even though often called 'calcium titanate', does not have distinct titanate ions. Perovskite structure (Fig. 1. depicts a corner-sharing network of TiO6 octahedra (necessarily the ReO3 structure;) with Ca2+ occupying the large central site coordinated through 12 oxygen ions.
Though, this division is not absolute and the varied structures of silicates provide instances of intermediate cases. ZrSiO4 (zircon) has discrete ions SiO44- but silicates like CaSiO3 do not contain individual SiO32-units but are created from tetrahedral SiO4 groups sharing corners to make rings or infinite chains.
Additional sharing of corners can make two- dimensional and three- dimensional networks. The distinct structures of silicates and carbonates reflect some typical and very significant differences in bonding preference between periods 2 and 3 in the p block.
Complex oxides are generally found when a nonmetal is exist, with oxoanions like nitrate NO3-,carbonate C32- phosphate P43-or sulfate SO42- but are also occasionally formed by metals in high oxidation states (example permanganate MnO4- in KMnO4). When a compound consists of two metallic elements the mixed oxide form is more normal but it is significant to notice that the compound formula itself gives very little guide to the structure (compare CaCO3 and CaSiO3 above). A identical structural variety is found with complex halides. For instance, the K2NiF4 structure is based on layers of corner-sharing NiF6 octahedra with no discrete complex ions, where K2PtCl4 consists of individual square planar ions [PtCl4]2-. These variations reflect the bonding preferences of NiII and PtII.