Intercalation and insertion compounds
Bromine and Alkali metals react with graphite to create solids known as intercalation compounds, in which the foreign atoms are inserted among the intact graphite layers. Several other layered solids, for instance dichalcogenides like TaS2, which have structures identical to CdI2, will also form intercalation compounds. The inserted species might be alkali metals or electron donor molecules like Organometallic or amines compounds. Occasionally compounds of definite composition may be created, like KC6 or C8Br, but in other cases intercalated phases may be nonstoichiometric, like LixTiS2 (0<x<1). Most intercalation reactions involve electron transfer among the guest and the host and change the electronic properties.
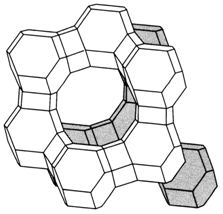
Fig. Representation of the structure of faujasite (see text).
The word insertion compound is employed for solids where atoms or ions enter a three-dimensional framework without disrupting its necessary structure. Several oxide bronzes are of this type, are based on transition metal oxides with inserted alkali or other electropositive metals. For instance, the sodium tungsten bronzes are of composition NaxWO3, in which x can range from zero up to about 0.9. Their structures are based on ReO3 framework with Na taking the large vacant site. So the structure resembles that of perovskite (Fig. 1. apart from that the site occupied by Ca in CaTiO3 is only partially occupied in NaxWO3. like with intercalation, electron transfer is also involved and NaxWO3 has a metallic appearance and good electronic conductivity where pure WO3 is a pale yellow insulator.
As described in Topic B6 earlier the synthesis of solids often needs high temperatures, due to the slow diffusion of atoms. Though, In intercalation compounds and some insertion compounds diffusion of guest species is more facile, and such type of compounds can frequently be made prepared under quite mild conditions, sometimes termed as chimie douce ('gentle chemistry'). Graphite's intercalation compounds of can be made directly through exposure of the solid to Br2 or to alkali metal vapors. Insertion compounds of lithium (that is small and diffuses rapidly in several hosts) can be prepared by reaction with n-butyl lithium. For an instance:
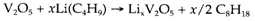
Insertion compounds of hydrogen like HxMoO3 can also be prepared, either through direct reaction of the host with H2 in the existence of a platinum catalyst, or through reduction with metallic zinc in aqueous acid. The structural features are distinct from those consisting of alkali metals. One would not suppose the very small H+ ion to occupy an intersitial site in similar way like a metal cation but rather to form a covalent bond with oxygen. Methods like IR spectroscopy do indeed show the presence of OH groups so that the compound above should be formulated as MoO3-x(OH)x.