Equilibrium constants
A complex generally is any species formed by particular association of molecules or ions through donor-acceptor interactions. In aqueous solution the most significant complexes are those cretaed among a metal cation and ligands, that may be ions (examples halides, cyanide, oxalate) or neutral molecules (examples ammonia, pyridine). The ligand works as a donor and change one or more water molecules from the main solvation sphere, and so a complex is distinct from an ion pair, that forms by purely electrostatic interactions in solvents of low polarity. Even though complex formation is particularly characteristic of transition metal ions it is through no means confined to them.
Various steps of complex formation might be possible and the successive equilibrium is remain constants for the reactions
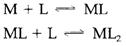
and so on are termed as the stepwise formation constants K1, K2.... The complete equilibrium constant for the reaction
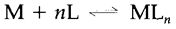
is given by
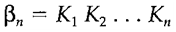
Stepwise successive formation constants frequently decrease regularly K1>K2>...of the maximum value being determined through the number of ligands which can be accommodated: this is frequently six other than for chelating ligands. The reduces can be understood on entropic (statistical) grounds, such as each successive ligand has one less place existing to attach. Exceptional influences may result from the size and charge of ligands and a reversal of the usual sequence can occasionally be attributed to particular electronic or structural influences. It is significant to remember that each ligand replaces one or more solvating water molecules. For instacne, in the Cd2+/Br- system K4>K3 like the octahedral species [Cd(H2O)3Br3]- is converted to tetrahedral [CdBr4]2- by an entropy gain resulting from the improved freedom of 3 water molecules. Soft and Hard behavior For cations created by metals in early groups in the periodic table the complexing strength with halide ions follows the sequence
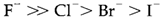
Where with some later transition metals and several post-transition metals the opposite sequence is found (for example Pt2+, Hg2+, Pb2+). The previous behavior is termed as class a and the later as class b behavior, and the variation is an instance of hard and soft properties, correspondingly. Class b ions create strong complexes with ligands like ammonia, that are softer than water, where class a ions do not complex with such type of ligands appreciably in water. Solvation plays an necessary part in understanding the issues behind class a and b behavior. Trends in bond strengths depict that approximately every ion would follow the class a sequence in the gas phase and the behavior in water is a partly a result of the weaker solvation of larger anions. With a class b ion like Hg2+ the bond strengths reduce more slowly in the sequence Hg-F>Hg-Cl>... than in the solvation energies of the halide ions. With a class a ion like Al3+, alternatively, the alteration in bond strengths is more marked than that in the solvation energies.
In solution the variation among the two classes is often manifested in dissimilar thermodynamic behavior. Class b complex creation is enthalpy dominated (that is driven by a negative ΔH) where class a formation is frequently entropy dominated (driven by positive ΔS). The strongest class a ions are highly and small charged (example. Be2+, Al3+) and have extreme negative entropies of solvation. Complexing with small highly charged ions like F- reduces the overall charge and therefore frees up water molecules, that are else ordered through solvation. Hard cations that have low charge/size ratio, like alkali ions, create very weak complexes with all ligands apart from macrocycles.
Some polyatomic ions like NO3-, CIO4-and PF6- have extremely low complexing power to either class a or b metals. They are helpful as counterions for studying the thermodynamic properties of metal ions (example electrode potentials;) not affected by complex formation.