Chelates and macrocycles
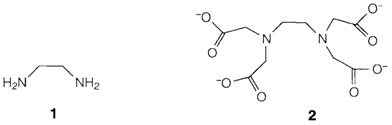
Chelating ligands are ones with two or more donor atoms sble of attaching concurrently to a cation: they are explained as bidentate, tridentate,...as per the number of atoms able of binding. Chelating ligands involve bidentate ethylenediamine (1) and ethylenediamine tetraacetate (EDTA, 2), that is hexadentate, containing two nitrogen donors and four oxygens (one from each carboxylate). Chelating ligands usually form stronger complexes than unidentate ones with the same donor properties. They are helpful for analysis of metal ions by complexometric titration and for removing toxic metals in cases of poisoning.
The basis of the chelate influence is entropic. Each ligand molecule can swap more than one solvating water molecule, so giving a favorable entropy increase. Structural needs occasionally subvert the effect: for instance, Ag+ does not depict the expected increase of K1 with ethylenediamine compared with ammonia, since it has a strong bonding preference for two ligand atoms in a linear configuration, that is structurally not possible with the bidentate ligand. The length of the chain created among ligand atoms is significant in chelate formation, the stable complexes usually being formed with four atoms (that is including the donors) thus that with the metal ion a five-membered ring is cretaed. Smaller ring sizes are less favorable due to the bond angles involved, larger ones due to the improved configurational entropy of the molecule (coming from free rotation concerning to the bonds), that is lost in forming the complex. Limiting the probability of bond rotation raises the complexing power even with optimum ring sizes, thus that phenanthroline (3) creates stronger complexes than bipyridyl (4).

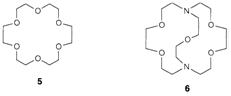
Reducing the configurational entropy is significant in macrocyclic ligands, in which several donor atoms are already 'tied' through a molecular framework into the optimal positions for complex formation. Instances are the cyclic crown ethers (example 18-crown-6, 5) and bicyclic cryptands (example [2.2.1]-crypt, 6). As supposed, complexing strength is improved, and the resultant macrocyclic effect allows complexes to be formed with ions like those of group 1, otherwise that have very low complexing power. Other characteristic of macrocyclic ligands is the size selectivity subsequent to dissimilar cavity sizes. So with ligand 6 complex stability follows the order Li+<Na+>K+>Rb+ and the selectivity can be changed through varying the ring size. Chelating and macrocyclic influences are significant in biological chemistry. Metal binding sites in metalloproteins consist of various ligand atoms, with suitable electronegativities, and arranged in a appropriate geometrical arrangement, to optimize the binding of a particular metal ion.