Structure
The major structure of every polypeptide in collagen is characterized through a repeating tripeptide sequence of Gly–X–Y where X is frequent but not exclusively, Y and Pro is often Hyp. Every of the three polypeptide chains in collagen is some
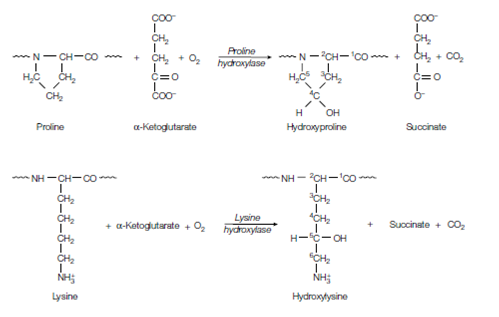
Figure: Formation of hydroxyproline and hydroxylysine.
1000 residues long and they each fold up into a helix which has only 3.3 residues per turn, certain than the 3.6 residues per turn of an α-helix. This secondary structure is distinctive to collagen and is frequently called the collagen helix. The three polypeptide chains lie wind round and parallel one another with a slight right-handed rope-like twist to form a triple-helical cable. Each third residue of every polypeptide passes by the center of the triple helix, that is so crowded which is only the small side-chain of Gly can ?t in. This describes the absolute needs for Gly at every third residue. This residues in the X and Y positions are situated on the outside of the triple-helical cable where there is room for the bulky side-chains of Pro and other residues. These three polypeptide chains are also staggered so in which the Gly residue in one chain is aligned with the X residue in the second and the Y residue in the third. The triple helix is held together through an extensive network of hydrogen bonds, in particular among the primary amino collection of Gly in one helix and the major carboxyl set of Pro in position X of one of the another helices. Additionally, the hydroxyl collection of Hyp residues participates in stabilizing the structure. The relatively in?exible Hyp and Pro also confer rigidity on the collagen structure.
The value of Gly at every third residue is shown when a mutation in the DNA encoding Type I collagen leads to the incorporation of a various amino acid at just one position in the 1000 residue polypeptide chain. For instance, if a mutation leads to the incorporation of Cys in the place of Gly the triple helix is disrupted as the -CH2-SH side-chain of Cys is too huge to ?t in the interior of the triple helix. This process leads to a partly unfolded structure which is susceptible to excessive glycosylation and hydroxylation and is not ef?ciently secreted through the ?broblast cells. In this, turn results in a defective collagen structure which can provide rise to skeletal deformities and brittle bones. A overall spectrum of like mutations are known that cause the production of defective collagen and give the result in osteogenesis imperfecta or brittle bones.
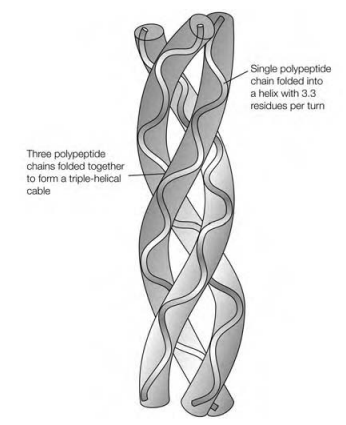
Figure: Arrangement of the three polypeptide chains in collagen.