Secretion and aggregation
When the collagen polypeptides are synthesized they have further amino acid residues (100–300) on both their C and N termini which are absent in the mature collagen ?ber in figure. These extension peptides frequently contain Cys residues, that are commonly absent from the remainder of the polypeptide chain. The addition peptides help to align properly the three polypeptides as they come together in the triple helix a procedure which may be aided through the formation of disul?de bonds among extension peptides on neighboring polypeptide chains. The addition peptides also prevent the premature aggregation of the procollagen triple helices within the cell. On secretion out of the ?broblast the addition peptides are erased through the action of extracellular peptidases that are shown in Figure. The resulting tropocollagen molecules then aggregate together in a staggered head- to-tail arrangement in the collagen ?ber.
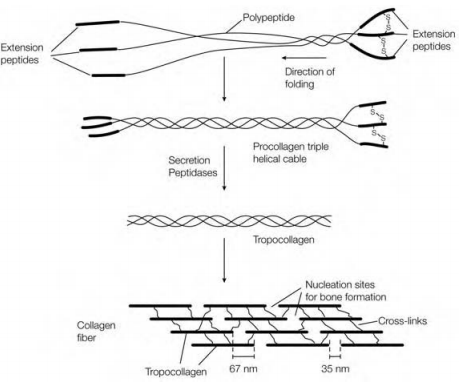
Figure: Role of the extension peptides in the folding and secretion of procollagen. Once secreted out of the cell, the extension peptides are removed and the resulting tropocollagen molecules aggregate and are cross-linked to form a ?ber.