Artificial radioactive decay series:
Isotopes are a set of nuclides whose atomic numbers (Z) are same but these have different mass numbers (A). These may be stable and radioactive. For example H has three isotopes, of which 1H and 2H are stable and 3H is radioactive. Isobars are nuclides having the same mass number (A) but different atomic numbers (Z). Thus 12B, 12C and 12N have 5, 6 and 7 protons respectively.
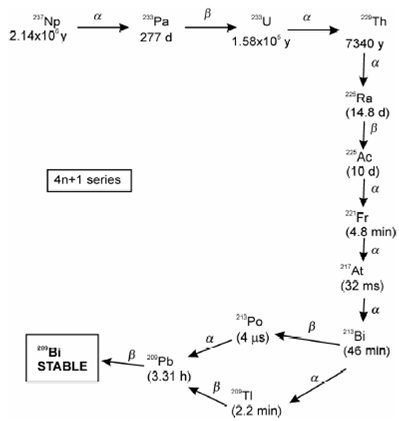
Figure: Artificial radioactive decay series of 237Np also called (4n+1) neptunium series