Particles of Matter:
The idea which matter exists in the form of particles, instead of as a continuous mass, is many centuries ago. How do we elucidate the fact that some substances are much denser than others, that some retain their shape whereas others flow freely, and that some are visible whereas others are not? The numerous ways in which matter can exist are hard to explain in any other way than by means of a particle theory. This was the logic ancient alchemists used when they imagined that matter is comprised of minute, invisible particles, or atoms.
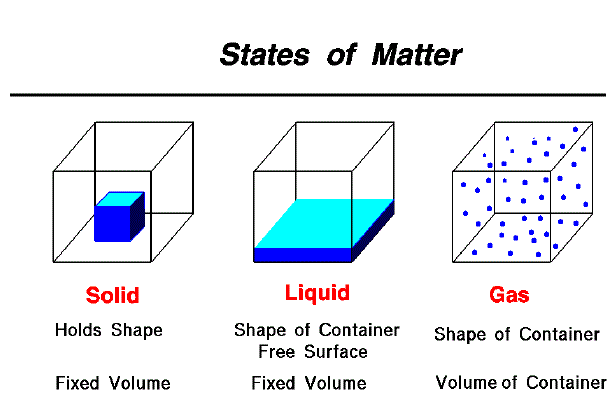
All atoms are made up of numerous smaller particles whizzing around. These subatomic particles are dense, though matter is generally empty space. Matter seems solid and continuous since the particles are too small that we cannot see them, and they move too fast that their individual motions would emerge as a blur even when the particles themselves could be seen. Though, the spaces within atoms are hugely greater than the particles which comprise them. When we could shrink ourselves down to the subatomic scale and also slow down time in proportion, a piece of metal would look somewhat like a huge, hysterical swarm of gnats. Did the first physical and chemical scientists realize that the atoms they had dreamed up actually consisted of smaller particles, that these particles in return consisted of tinier ones still, and which some people in future generations would come to believe that the particle series expands down to smaller and smaller scales ad infinitum?