Representation of the anisotropic polarisability of a molecule:
A weaker bond along with loosely held electrons is more polarisable; a single bond is more polarisable than a double bond among the similar set of atoms. In addition, the polarisability could be anisotropic, that is the electrons of a bond might be polarised to different extents while the field is applied in different directions. For instance, the hydrogen molecule gets polarised to different extents while the field is applied in the direction of the bond or in the direction perpendicular to it, as shown in Figure.
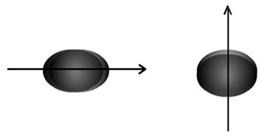
Figure: Schematic representation of the anisotropic polarisability of a molecule
What would happen if we place a molecule within an oscillating electric field such as that of an electromagnetic radiation? Let us see.
The electric field related along with a radiation having a frequency νex varies as per the following equation.
E = E0 cos( 2πν ext)
where E is the electrical field strength at any time t and E0 is the amplitude of the wave. While such a radiation is made to interact along with a molecule it would induce a dipole moment in it. Additionally, as the electrical field of the radiation is oscillating the resulting induced dipole moment would also oscillate at the similar frequency as that of the radiation. Which is,
µ = αE = αE0 cos( 2πν ext)
This oscillating dipole would immediately produce a radiation of the frequency, ν ex and we have a ready explanation for Rayleigh scattering.