Patch clamping
Patch clamping is an in vitro methods which makes it possible to study the electrophysiology of single ion channels. It works through forming a very high electrical resistance seal among a glass micropipette and the surface of a cell. Only currents flowing by the patch under the electrode will be recorded. This will authorize the extremely small currents which flow by single ion channels (about 1 pA) to be measured. The electronics permit the voltage of the patch to be clamped so voltage-clamping experiments can be performed.
There are various configurations of patch clamping, each useful for particular kinds of experiment that are given below:
- Cell attached. Cell attached is used for measuring single channel currents in intact cells. 2nd-messenger-induced modifications of the patched channels can be investigated in response to bathing the cell with specific agents, for instance neurotransmitters.
- Whole cell. In whole cell configuration the patch under the microelectrode is ruptured. The current flowing by the electrode represents the sum of all the currents flowing by individual ion channels on the cell surface. Therefore overall cell patching measures macroscopic currents.
- Outside-out. Outside out is one of two patch clamp modes used to study single-channel currents in that the patch is erased from the cell but remains sealed to the pipette tip. This configuration is used to learning the effects of ligands like as hormones, neurotransmitters or externally acting drugs on the channels. Those ligands are added to the bath because bath solutions can be changed much more simply and rapidly than the pipette solution. This has obvious benefits for performing complicated experiments like as investigating dose–response relationships.
- Inside-out. This is the second of the patch-only configurations, commonly used for the detailed examination of second messengers because these can be applied directly to the inside face of the membrane by the bath solution.
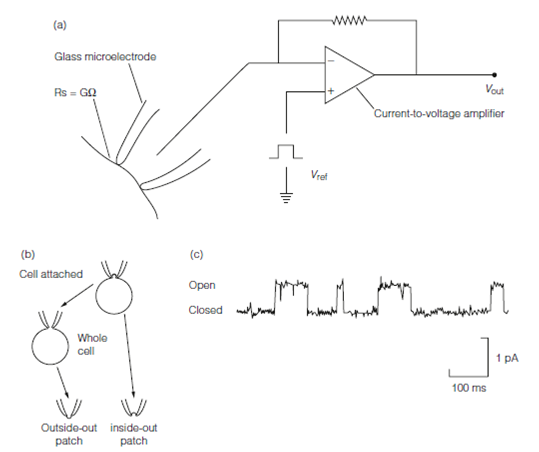
Figure: Patch clamping. (a) The circuitry, Vref is used to clamp the potential of the neuron membrane, the method relies on forming a high resistance seal, Rs between the pipette tip and the cell membrane. (b) Patch clamp configurations. (c) Single channel currents through a GABAA receptor.
A representative patch clamp recording is described in Figure. Every of the square wave events is the opening of a particular channel. The height of the wave is the unitary channel current; the duration of the wave is the time for that it is open. Statistical analysis of huge numbers of opening events gives estimates for parameters like as mean channel open time and permits models of channel kinetics to be tested. Like studies are very useful in fathoming out how neuroactive drugs act at the molecular level.