Claisen condensation of two different esters:
Two dissimilar esters can be employed in the Claisen condensation as long as one of the esters has no α-protons and cannot make an enolate ion.
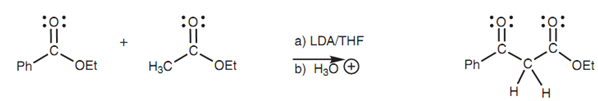
Figure: Claisen condensation of two different esters.
From the mixed Claisen condensation of a ketone with an ester the β-Diketones can be synthesized. Once again, it is advisable to use an ester that cannot form an enolate ion to prevent competing Claisen condensations.
In both these last two instances, a very strong base is employed in the form of LDA such that the enolate ion is created quantitatively (from ethyl acetate and acetone correspondingly). This avoids the chance of self-Claisen condensation and limits the reaction to the crossed Claisen condensation.
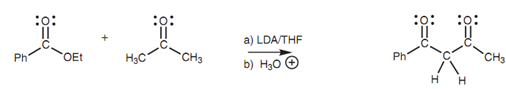
Figure: Claisen condensation of a ketone with an ester.