Thermogravimetric analysis:
Thermogravimetric analysis (TGA) is the most widely used thermal method. That is based on the measurement of mass loss of material as a function of temperature. Within thermogravimetry a continuous graph of mass modifies against temperature is acquired while a substance is heated at a uniform rate or kept at constant temperature. The plot of mass change versus temperature (T) is referred to as the thermogravimetric curve (TG curve). For the TG curve, we generally plot mass (m) decreasing downwards on the y axis (ordinate), and temperature (T) increasing to the right on the x axis (abscissa) as described in Figure. Sometime we might plot time (t) within place of T. TG curve helps within revealing the extent of purity of analytical samples and in determining the mode of their transformations within specified range of temperature.
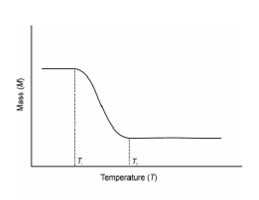
Figure: A Typical TG Curve
In thermogravimetry, the word 'decomposition temperature' is a finished misnomer. Within a TG curve of a single stage decomposition, there are two features temperatures; the initial Ti and the last temperature Tf. Ti is described as the lowest temperature at that the onset of a mass change could be detected through thermo balance operating under particular conditions and Tf as the final temperature at that the particular decomposition appear to be complete. Although Ti has no fundamental significance, it could still be meaningful features of a TG curve and the term procedural decomposition temperature has been suggested. The difference Tf - Ti is termed as reaction interval. In a dynamic thermogravimetry a sample is subjected to continuous increase in temperature commonly linear along with time whereas in isothermal or static thermogravimetry the sample is managed at a constant temperature for a period of time during that any change in mass is remembered. Now we will take up the instrumentation generally used to gain TG Curve.