Metallic Bond
Metallic bonding occurs only in metallic elements. Metals have few electrons in their outermost shell. Thus they can easily lose these electrons to their surrounding (sea of electrons), leaving the atoms as positive ions (cations). The electrons acts like a glue to hold the positively charged ions together. This electromagnetic attraction between delocalized electrons and the metallic ions is known as metallic bond.
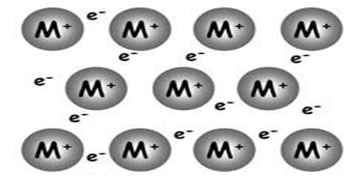
Metallic bond accounts for most of the properties seen in metals, like electricity and heat conduction, malleable, ductile, high density, high strength etc.
Properties of metals:
(i) Good conductors of heat and electricity
(ii) Solids at room temperature and pressure (except mercury)
(iii) High melting and boiling points
(iv) Ductile (can be drawn into wires)
(v) Malleable (can be beaten into sheets)
(vi) High density
(vii) Lustrous (shiny when polished)
(viii) Sonorous (produces sounds when struck)
(ix) Always forms positive ions