Hydrogen Bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom like nitrogen, oxygen and fluorine. When a hydrogen atom is covalently bonded to a highly electronegative atom (esp N2 O2 and F2), then the electron cloud, that was supposed to be shared equally between the two atoms, is pulled towards the electronegative atom. This causes a slight negative charge on the electronegative atom and a slight positive charge on the hydrogen atom. This slight charge causes attraction between hydrogen atom of one molecule to the electronegative atom of another molecule. This attraction is known as hydrogen bond.
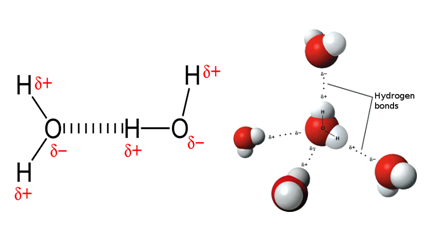
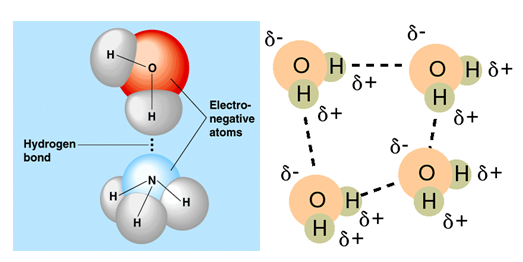
Although a single a hydrogen bond is a weak force, hydrogen bonding between all the molecules makes a significant impact on the physical state of a compound. For example, water is a liquid with considerably high boiling point because of presence of hydrogen bond and hydrogen sulphide, a compound similar to water, is a gas at room temperature.
Dipole-dipole interactions:
It is similar to hydrogen bond except it may not involve hydrogen. When two atoms of significantly different electro negativity are covalently bonded together, the electrons are pulled towards the more electronegative atom giving itself a slight negative charge and its partner atom a slight positive charge. This gives result to the attraction between opposite charges on 2 different molecules.
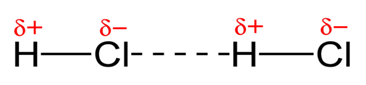
An instance is the attraction among molecules of hydrogen chloride. The attraction is not hydrogen bond because electronegativies are not different enough.