Covalent bond
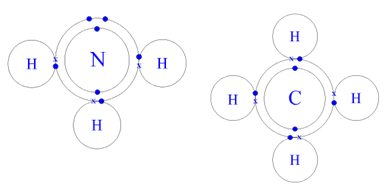
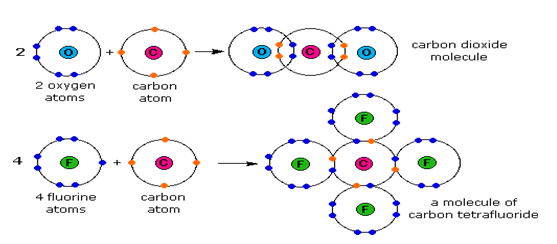
Covalent bonding occurs only between atoms of nonmetals. It involves sharing of electrons between the atoms to fulfill the outermost shell of one another.
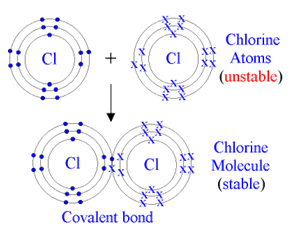
For example, chlorine has 7 electrons in its outermost shell, requiring one more electrons to complete its outermost shell. In a gas jar full of chlorine one chlorine atom reacts with another and each one shares one electron with the other one, thus mutually fulfilling one another's outermost shell. So they gain a stable structure forming a chlorine molecule.
This is why most gases (except noble gases) exist as molecules rather than individual atoms. Covalent bonding also occurs between different nonmetallic atoms to form compounds.
Properties of covalent compounds:
(i) They are composed of molecules.
(ii) Covalent compounds are usually liquid or gas with low melting and boiling points.
(iii) They are nonconductors of heat and electricity in any state.
(iv) They are usually insoluble in water but soluble in nonpolar solvents like benzene and alcohol.
(v) They tend to be more flammable than ionic compounds.
(vi) Chemical reactions involving covalent compounds tend to be slow.