Chromatography
A mixture of several components enters a chromatography process, and the different components are flushed through the system at different rates. The differential rates of migration as the mixture moves over the adsorptive materials provide separation. Repeated sorption or desorption acts which take place during the movement of sample over the stationary bed determine the rates. Smaller the affinity a molecule has for the stationary phase, shorter the time spent in the column. Methods of chemical analysis are truly specific for a particular analytic. It is found that the analytic of interest should be separated from the myriad of individual compounds which may be present in a sample. As well as providing the analytical scientist along with the methods of separation, chromatographic techniques can provide methods of analysis also.
Chromatography involves the sample being dissolved in the mobile phase. The mobile phase is forced through an immobile, stationary phase. The phases are chosen so that components of the sample have differing solubility’s in every phase. Chromatography is a physical methods of separation in which components to be separated are distributed between 2 phases, 1 of which is stationary while the other moves in a particular direction. The eluent is the mobile phase leaving the column.
A component which is soluble in the stationary phase will take longer to travel through it than the component which is not soluble in the stationary phase but soluble in the mobile phase. As a result of these differences in the motilities, sample components will become separated from each other as they travel through stationary phase. To obtain optimal separations, sharp, symmetrical chromatographic peaks should be obtained. This means that band broadening should be limited. It is beneficial to measure the efficiency of the column. A sample of blood can be analyzed by this technique.
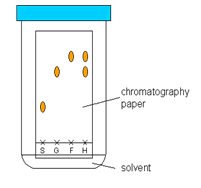
CHROMATOGRAPHY technique of separating two or more colors from a mixture of colors. first a rectangular filter paper is taken & a base line is drawn near one end of the paper with the pencil. Spots of the mixtures of the colors are put on the base line. Then the paper is hung over an organic solvent, such as alcohol, in such a way so that the edge of the paper is touching the solvent. It should be ensured that the base line never touches solvent.
The solvent will travel up the paper and on its way it will dissolve the mixtures of colors. As the mixtures of colors travels up they will be separated. Because different compounds have different affinity (adsorption) for the filter paper. Those with higher adsorption will spread slower than those with lower adsorption. Thus the colours will be separated.