pH:
The purpose for controlling pH within the reactor coolant system is to minimize and control corrosion. As earlier discuss the presence of excess H+ ions in solution output within an acidic condition. Within reactor facilities (except those hold aluminum components), acidic conditions are detrimental to the materials of construction inside a number of ways. An acidic condition within the main coolant output in processes those are potentially harmful to the system as follows. First, a low pH promotes rapid corrosion through deteriorating or "stripping off" the protective corrosion film, and other one, corrosion products like as ferrous oxide (Fe3O4), which is predominant in the corrosion film, are extremely soluble in an acidic solution. Below figure display how the corrosion rate increases as the pH decreases. Therefore for facilities not using aluminum elements, a neutral or highly basic pH is less corrosive.
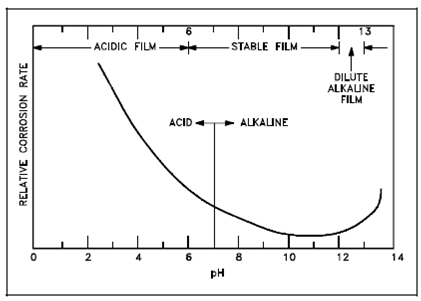
Figure: Corrosion Rate vs. pH for Iron
In nuclear facilities that do not use chemical shim to control reactivity, pH is generally managed at an associatively high value, like as a pH of about 10. In those facilities the upper limit for pH is set based on caustic stress corrosion considerations since caustic stress corrosion becomes more possible as higher pH values are approached.
In facilities which use chemical shim reactivity control (chemical shim includes the addition of boron within the form of boric acid) the pH is managed at a much lower value. A low pH is essential since of the huge amounts of boric acid added to the reactor coolant. Therefore, pH in these facilities is managed as high as possible consistent along with the reactivity needs of the nuclear facility, along with pH range from 5 to 7 being general.
In facilities using aluminum components, pH is managed on the acidic side of the scale since of the corrosion features of aluminum discussed within Module 2. In these facilities pH might be controlled through the addition of a dilute nitric acid (HNO3) solution to the reactor coolant system within conjunction along with an ion exchange system of a few categories.
Regardless of the pH range maintained, many facilities use an ion exchange process (described in Module 4) to help control pH. For the high pH facilities, a most common means of control is the use of lithium or an ammonium form cation and a hydroxyl form anion. When lithium is used, it must be 7Li because other lithium isotopes produce tritium that represents a significant biological hazard to personnel. Within facilities that employ high pH chemistry control and do not use chemical shim reactivity control, it is sometimes essential to add a strong base solution such as ammonium or lithium hydroxide. While chemical additions are used for pH control, facility design and operating process are utilized to preclude overconcentration at any point in the system that might lead to caustic stress corrosion conditions. Several reactions that take place in the reactor coolant system can affect pH; therefore chemistry control must be considered carefully to preclude upsetting the pH balance provided by the ion exchanger.