Hydrogen:
Since the presence of dissolved oxygen contributes too many mechanisms of corrosion, a concentration of oxygen is controlled and decreased through the addition of scavenging agents in most facilities. Hydrogen gas (H2) and hydrazine (N2H4) are the scavenging agents generally used to eliminate dissolved oxygen from the reactor coolant system. Those substances scavenge oxygen through the following reactions.
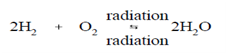 (3-13)
(3-13)
N2H4 + O2 → 2 H2O + N2 (3-21)
Since hydrazine decomposes rapidly at temperatures above about 200º F (building NH3, H2, and N2), hydrogen gas is used as the scavenging agent in during hot operation and hydrazine is used when the reactor coolant system is cooled below 200oF.
(Heat)
2 N2H4 →2NH3 + N2 + H2
(decomposition of hydrazine)
The decomposition reactions of hydrazine pose further problems in chemistry control. Even if enough hydrazine were added to overcome the loss because of decomposition, instability of coolant pH would possibly occur through the following reactions.
2 N2 + 5 O2 + 2 H2O → 4 HNO3 (acid) (3-16)
3 H2 + N2 + 2 H2O → 2 NH4OH (base) (3-22)
The use of hydrogen gas at temperatures above 200ºF precludes the generation of the compounds formed through Reactions (3-16) and (3-22). Further, hydrogen is compatible along with the high flux levels present in the reactor core. Accordingly, benefits might be taken of the reversibility of the radiolytic decomposition of water. The subsequent reaction describes the scavenging procedure utilizing hydrogen.
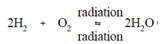 (3-13)
(3-13)
As denoted, the reaction is an equilibrium procedure and will thus depend on the associative concentrations of the reactants and the products. Through manages an excess of hydrogen (H2), the reaction is forced to shift to the right and theoretically eliminates some dissolved oxygen which might be present. As long as an inventory of H2 is present within the coolant, dissolved oxygen will be eliminated or forced to recombine instantly after radiolytic decomposition, thus being unavailable for corrosion reactions.