Reactions:
Nitriles (RCN) are hydrolyzed to carboxylic acids (RCO2H) in acidic or basic aqueous solutions. The mechanism of the acid-catalyzed hydrolysis includes initial protonation of the nitrogen atom of nitrile. This activates the nitrile group in the direction of nucleophilic attack by water at the electrophilic carbon. One of the nitrile π bonds breaks concurrently and both the π electrons move onto the nitrogen resultant in a hydroxy imine. This fast isomerizes to a primary amide that is hydrolyzed within the reaction conditions to provide the carboxylic acid and ammonia.
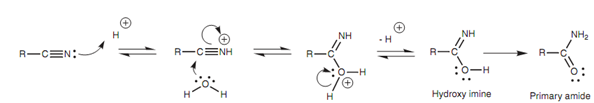
Figure: Acid-catalyzed hydrolysis of nitrile to carboxylic acid.
Nitriles (RCN) can be get reduced to primary amines (RCH2NH2) with lithium aluminum hydride that gives the equivalent of a nucleophilic hydride ion. The reaction can be described by the nucleophilic attack of two hydride ions.
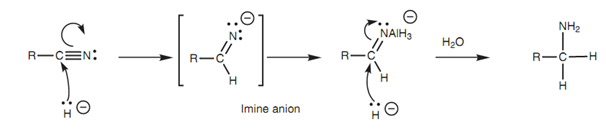
Figure: Reduction of nitriles to form primary amines.
With a milder reducing agent like DIBAH (diisobutylaluminum hydride), the reaction prevents after the addition of one hydride ion, and an aldehyde is acquired instead (RCHO).