Properties:
In shape the nitrile group (CN) is linear with both the carbon and the nitrogen atoms being sp hybridized. The triple bond linking the two atoms contains one σ bond and two π bonds. Nitriles are much strongly polarized. The carbon is an electrophilic center whereas the nitrogen is a nucleophilic center. Nucleophiles react with nitriles at the electrophilic carbon. Generally, the nucleophile will form a bond to the electrophilic carbon resultant in simultaneous breaking of one of the π bonds.
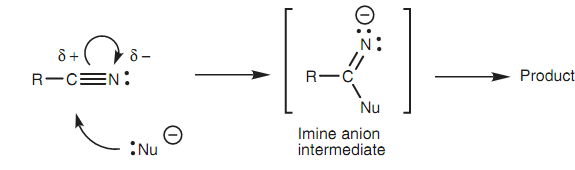
Figure: Reaction between nucleophile and nitriles.
The π electrons end up on the nitrogen to make a sp hybridized imine anion that after that reacts further to give different products depending upon the reaction conditions used.