Chemical Precipitation
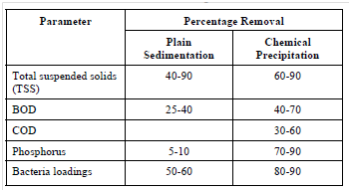
Chemical coagulation of raw wastewater before sedimentation promotes the flocculation of finely divided solids into more readily settleable flocs, thus enhancing the effectiveness of suspended solid, BOD and phosphorus removal as contras to plain sedimentation without coagulation (Table 3). The degree of clarification acquired depends on the quantity of chemicals used and the care along with that the process is controlled.
Table 3: Removal Efficiency of Plain Sedimentation vs Chemical Precipitation
Coagulant selection for enhanced sedimentation is based on performance, reliability and value. Performance evaluation uses jar tests of the actual wastewater to determine dosages and effectiveness. Chemical coagulants that are generally used in waste-water treatment include alum (Al2 (SO4)3.18.3 H2O), ferric chloride (FeCl3.6H2O), ferric sulphate (Fe2(SO4)3), ferrous sulphate (FeSO4.7H2O) and lime (Ca(OH)2). Organic polyelectrolytes are a few times used as flocculation aids. Suspended solids removal by chemical treatment involves a series of three unit operations: rapid mixing, flocculation and settling. First, a chemical is added and fully dispersed throughout the wastewater by rapid mixing for 20-30 seconds in a basin with a turbine mixer. The Coagulated particles are then brought together via flocculation by mechanically inducing velocity gradients inside the liquid. Flocculation takes 15 to 30 minutes within a basin holding turbine or paddle-type mixers. The final step is the clarification through gravity. A once-through chemical treatment system is illustrated in Figure 10.
The benefits of coagulation involve greater removal effectiveness, a feasibility of using higher overflow rates and more consistent performance. Instead, coagulation results in a larger mass of primary sludge which is frequent more hard to thicken and dewater. It also entails higher operational costs and demands greater attention on the part of the operator.
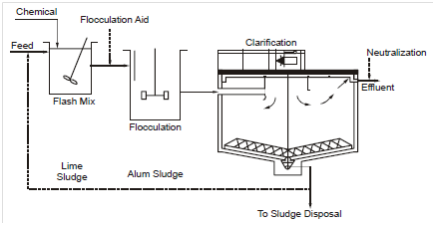
Figure 10: A Once-Through Chemical Treatment System