Shielding Mechanism:
The magnitude of the chemical shift is proportional to the strength of the magnetic field generated by the circulation of surrounding electrons about the proton. In order to understand the chemical shift value originating from structural arrangements, it is essential to understand the nature of this electron circulation and the corresponding induced magnetic area. The protons experience shielding because of the combination of different types of electronic circulations this is explained as follows.
It may be noted that electronic distribution for a free hydrogen atom and hydride ion are spherically symmetrical. An applied magnetic area induces electronic circulation about the nucleus in a plane perpendicular to the applied field. This generates a small magnetic field that, within the neighbourhood of the nucleus, is opposed to the direction of the applied field as schematically shown in Figure. This is called diamagnetic effect and causes the resonance to be observed at high field.
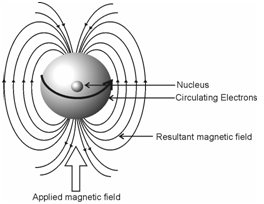
Figure: Diamagnetic shielding effect: A mechanism for chemical shift
In organic molecules where hydrogen is bound to carbon and other atoms, the electron distribution is not spherical as in the above case. The electrons occupy p and d orbitals in addition to the spherical s orbitals. Within such a case two kinds of effects are produced. These are diamagnetic effects in that the induced magnetic field direction is opposed to the applied magnetic field and shields the nucleus. The other kind of effect is paramagnetic effect and arises due to the induced magnetic field whose direction is parallel to the applied magnetic area. Those effects cause deshielding of the nucleus.