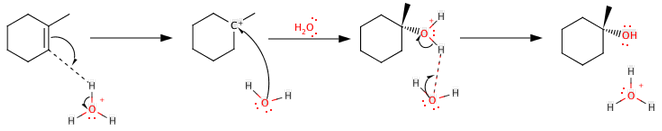
A hydration reaction is a organic chemical reaction in which a hydroxyl group (OH-) and a hydrogen cation (an acidic proton) are added to the two carbon atoms bonded together in the carbon-carbon double bond which creates an alkene functional group. The reaction generally runs in a strong acidic, aqueous solution. Hydration is different from hydrolysis in that hydrolysis cleaves the non-water component in two. Hydration left the non-water component intact.
The common chemical equation of the reaction is as follows:
RRC=CH2 in H2O/acid → RRC(-OH)-CH3
Reaction Mechanism -
Email based Hydration reaction assignment help - Hydration reaction homework help at Expertsmind
Are you finding answers for Hydration reaction based questions? Ask Hydration reaction questions and get answers from qualified and experienced chemistry tutors anytime from anywhere 24x7. We at www.expertsmind.com offer Hydration reaction assignment help - Hydration reaction homework help and Chemical Reactions problem's solution with step by step procedure.
Why Expertsmind for Chemistry assignment help service
- Higher degree holder and experienced tutors
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- On Time Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours