The Favorskii rearrangement (different from Favorskii reaction), named after Russian chemist Alexei Yevgrafovich Favorskii, is mainly a rearrangement of cyclopropanones and α-halo ketones which gives rise to carboxylic acid derivatives. In case of cyclic α-halo ketones, the Favorski rearrangement contains a ring contraction. This rearrangement occurs in the presence of a base, occasionally hydroxide, to give a carboxylic acid but most of the time either an alkoxide base or an amine to give an ester or an amide, correspondingly. α,α'-Dihaloketones remove HX under the reaction conditions to yield α,β-unsaturated carbonyl compounds.
In case of cylic α-halo ketones, the rearrangement takes place as shown below:
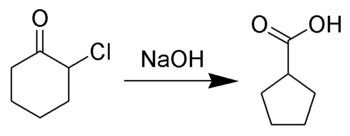
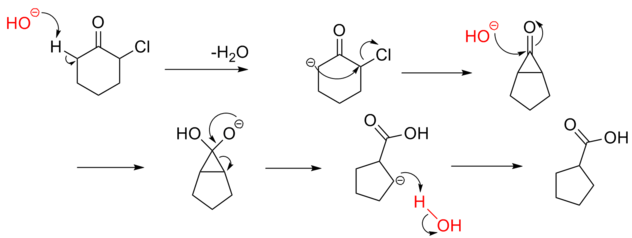
Reaction Mechanism -
Email based Favorskii rearrangement assignment help - Favorskii rearrangement homework help at Expertsmind
Are you finding answers for Favorskii rearrangement based questions? Ask Favorskii rearrangement questions and get answers from qualified and experienced chemistry tutors anytime from anywhere 24x7. We at www.expertsmind.com offer Favorskii rearrangement assignment help - Favorskii rearrangement homework help and Chemical Reactions problem's solution with step by step procedure.
Why Expertsmind for Chemistry assignment help service
- Higher degree holder and experienced tutors
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- On Time Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours