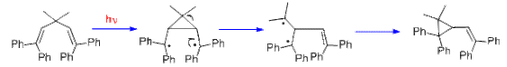
The di-pi-methane rearrangement is a photochemical reaction of a molecular entity including two π-systems, divided by a saturated carbon atom (a 1,4-diene or an allyl-substituted aromatic analog), to give an ene- (or aryl-) substituted cyclopropane. The rearrangement reaction properly amounts to a 1,2 transfer of one ene group (in the diene) or the aryl group (in the allyl-aromatic analog) and bond formation among the lateral carbons of the non-migrating moiety.
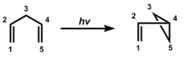
Reaction Mechanism-
Email based Di-pi-methane rearrangement assignment help - Di-pi-methane rearrangement homework help at Expertsmind
Are you finding answers for Di-pi-methane rearrangement based questions? Ask Di-pi-methane rearrangement questions and get answers from qualified and experienced chemistry tutors anytime from anywhere 24x7. We at www.expertsmind.com offer Di-pi-methane rearrangement assignment help - Di-pi-methane rearrangement homework help and Chemical Reactions problem's solution with step by step procedure.
Why Expertsmind for Chemistry assignment help service
- Higher degree holder and experienced tutors
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- On Time Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours