Metallic and non-metallic elements
The most significant classification of elements is that of metallic versus non-metallic. Metallic elements form solids which are good conductors of electricity and have structures with lots of near neighbours and where bonding is not strongly directional. Non-metallic elements create molecules or covalent solids, which are usually poor conductors of
electricity and where bonding is markedly directional in character. This difference on the basis of physical properties is fairly clear-cut and is displayed in the periodic table in diagram. 1. All elements of the s, d and f blocks are metallic (apart from hydrogen), non-metallic ones being confined to hydrogen and to the upper right-hand part of the p block. The most noticeable atomic parameter that determines this behaviour is electronegativity (particularly Fig. 1).
Various types of chemical behaviour are associated with the two types of element.
- General metallic elements are good reducing agents (for instance, reacting with water to produce dihydrogen) and form hydrated cations in aqueous solution (Na+, Mg2+, etc.). They have solid halides and oxides that are well explained by the ionic model. The oxides are basic and either react with water to generate hydroxide ions (OH-) or if insoluble under the neutral circumstances, dissolve in acidic solutions. Their hydrides are solids with some ionic (H-) character.
General non-metallic elements create ionic compounds with electropositive metals. They create anions in water, either monatomic (example Cl-) or oxoanions (example NO3-,SO42- ). They have molecular halides and hydrides. Their oxides are either polymeric or molecular covalent in structure and are acidic, reacting with water (like in halides) to gnerate oxoacids (H2CO3, H2SO4, etc.)
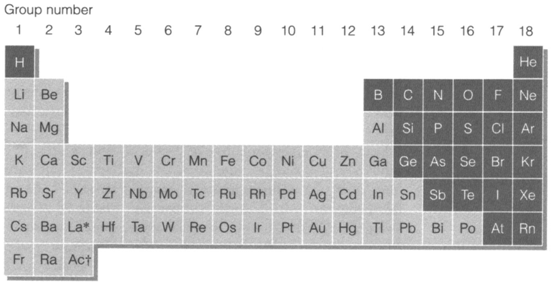
Fig. 1. Periodic table showing metallic and (heavily shaded) non-metallic elements.
It must be known that this categorized has many limitations and borderline behaviour is general. In addition to their general cationic behavior, several metallic elements form some compounds where bonding is predominantly covalent. Some form anionic species like MnO4- or even Na-. Several metals in later groups are much less electropositive than the typical definition would propose and the metal-nonmetal borderline in the p block involves a constant gradation in chemical behaviour rather than a discontinuous boundary. Nonmetallic elements close to the metallic borderline (that is Si, Ge, As, Sb, Se, Te) depict less tendency to anionic behavior and are sometimes called metalloids.