Hardness:
Hardness in water is defined as that characteristic which prevents formation of sufficient leather or foam when such water is mixed with soaps.
Hardness due to presence of bicarbonates and carbonates of calcium and magnesium are known as temporary hardness because such hardness is removed by simple boiling or by adding lime to water. Due to presence of sulphates, chlorides and nitrates of calcium or magnesium present in water the hardness does not go by boiling and it requires some special treatment for removing hardness or making it soft. Such hardness is known as permanent hardness. Hardness is commonly defined as the calcium carbonate equivalent of calcium and magnesium ions present in water and is expressed in mg/1. The hardness in mg/1 can be determined by determination of amounts of calcium and magnesium ions present in water by filtration.
Total hardness (mg/l) of CaCO3
= {Ca ++ in mg / l × Combining Weight of CaCO3 /Combining Weight of Ca ++}
+ {Mg++ in mg / l × Combining Weight of CaCO3 / Combining Weight of Mg ++}
The combining weight of Ca++, Mg++ and CaCO3 are 20, 12, and 50, respectively.
∴ Total Hardness = Ca ++ mg / l × 50/20 + Mg++ mg / l × 50/12
Sometimes, the hardness is expressed in degree of hardness. Each British degree of hardness is equal to 14.25 mg/1. Comparison of hardness level expressed in mg/1 as CaCO3 is given in Table.
Table : Comparison of Hardness
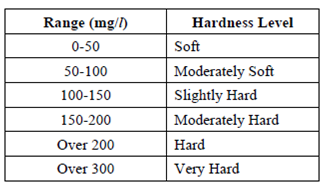
If the hardness is less than 150 mg/l, it is not economical to soften the water. The problems caused by excessive hardness give rise to formation of scale in boilers and hot water system. Temporary hardness to some extent is preferable, because water softer than 30-50 mg/l tends to be corrosive and is likely to take lead from pipes into the water supply.