Ionic Bonds:
An ionic bond is twisted while one or more electrons are wholly transferred from one part to another, and the elements are held together through the force of attraction because of the opposing charges. An instance of ionic bonding is displays in below figure for sodium chloride (table salt).
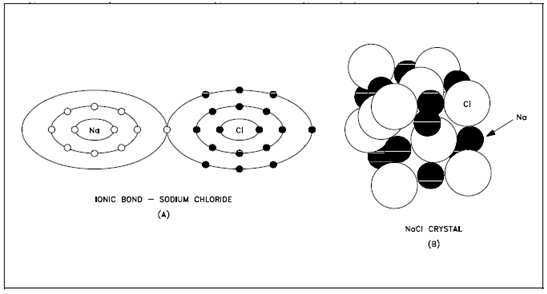
Figure: Ionic Bond, Sodium Chloride
The sodium atom loses the single electron within its outer shell to the chlorine atom that uses the electron to fill its outer shell. While this occurs, a sodium atom is left along with a +1 charge and the chlorine atom a -1 charge. The ionic bond is established as an output of the attraction of the two oppositely-charged particles. No single negatively-charged ion has a greater tendency to bond to a particular positively-charged ion than to some other ion.
Since of this, the positive and negative ions arrange themselves within three dimensions, as displays in above figure to balance the charges several ions. Within sodium chloride, for instance, every chloride ion is surrounded through as several sodium ions as could simply crowd around it, namely six. As same, every sodium ion is surrounded through six chloride ions. Thus, every chloride ion is bonded to a six nearest sodium ions and bonded to a lesser extent to the more distant sodium ions. Therefore, the ionic bond is a force holding several atoms or ions together rather than a bond among two individual ions or atoms.