The Mole:
A single atom or some atoms are rarely encountered. Alter, larger, macroscopic quantities are used to quantify or measure collections of atoms or molecules, a gallon of alcohol, like as a glass of water, or two aspirin. Chemists have introduced a huge unit of matter, a mole, to deal along with macroscopic samples of matter.
One mole represents a definite number of objects, substances, or particles. (For instance, a mole of ions, a mole of atoms, a mole of molecules, and even, in theory, a mole of elephants.) Mole is described as the quantity of a pure substance which contains 6.022 x 1023 units (ions, atoms, molecules, or elephants) of which substance. Alternatively, a mole is Avogadro's number of anything.
To any element, the mass of a mole of which element's atoms is the atomic mass expressed in units of grams. For instance, to compute the mass of a mole of copper atoms, easily express the atomic mass of copper within units of grams. Since the atomic mass of copper is 63.546 amu, the mole of copper has a mass of 63.546 grams. A value for the atomic mass of gold is 196.967 amu. Thus, a mole of gold has a mass of 196.967 grams. A mass of a mole of atoms is known as the gram atomic weight (GAW). The mole concept permits the conversion of grams of a substance to moles and vice versa.
Another figure contains a ball of gold and a ball of copper. The two balls are of various masses and different sizes, other than each contain an identical number of atoms.
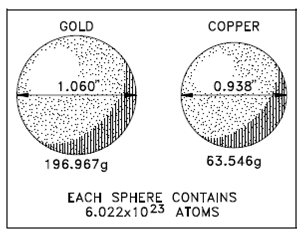
Figure: A Mole of Gold Compared to a Mole of Copper