Energy:
The energy of the electromagnetic radiation depends on its wavelength or frequency. The relationship is as under.
E = hν = hc/λ
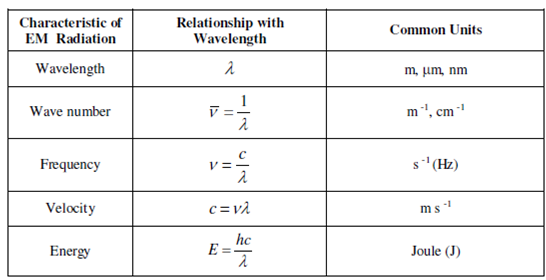
where, h is the Planck's constant and has a value of = 6.626 × 10 -34 J s, ν is the frequency c is the velocity and λ is the wavelength. As you could possibly make out that the energy is straight associated to the frequency and inversely related to the wavelength of the radiation. Instead a high frequency radiation will have a higher energy while a longer wavelength radiation will be low in energy. Table 1.1 summarizes the important characteristics of the EM radiation, their relationship with wavelength and the common units of their measurement.
Table: Characteristics of EM radiation, their relationship with wavelength and common units of measurement