Triple Point
Lastly, consider a preliminary pressure of the ice of 0.08854 psia. Again, as an outcome of heat transfer, the temperature will rise until it reaches 32°F. At this point, though, further heat transfer might outcome in some of the ice becoming vapor and some becoming liquid since it is probable to have the three phases in symmetry. This is termed as the triple point, stated as the state in which all three phases might be present in equilibrium.
Figure below is a pressure-temperature diagram for water which shows how the solid, liquid, and vapor phases might exist altogether in equilibrium. All along the sublimation line, the solid & vapor phases are in equilibrium, all along the fusion line, the solid and liquid phases are in equilibrium; and all along the vaporization line, the liquid and vapor phases are in symmetry. The only point at which all three phases might exist in equilibrium is the triple point. The pressure and temperature for the triple point of water are 32.02°F & 0.08865 psia. The vaporization line finishes at the critical point since there is no dissimilar change from the liquid phase to the vapor phase over the critical point.
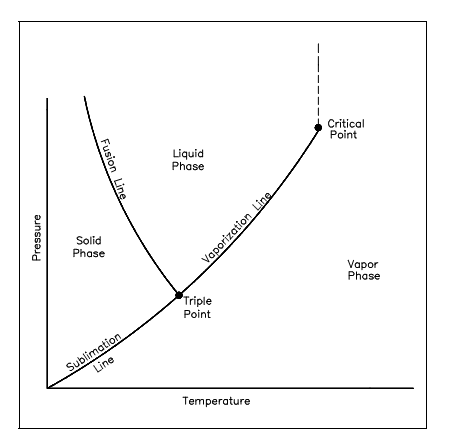
Figure: Pressure-Temperature diagram