Saturation
The word saturation defines a condition in which a mixture of liquid and vapor can exist altogether at a given pressure and temperature. The temperature at which vaporization (i.e., boiling) begins to take place for a given pressure is termed as the boiling point or saturation temperature. The pressure at which vaporization (i.e., boiling) begins to take place for a given temperature is termed as the saturation pressure. For water at 212°F, the saturation pressure is 14.7 psia and, for water is 14.7 psia, the saturation temperature is 212°F. For a pure substance there is a specific association among saturation temperature and saturation pressure. The high the pressure is, the higher the saturation temperature. The graphical presentation of this association among temperature and pressure at saturated conditions is termed as the vapor pressure curve. The usual vapor pressure curve is shown in figure below. The vapor or liquid mixture is at saturation whenever the cases of pressure and temperature fall on the curve.
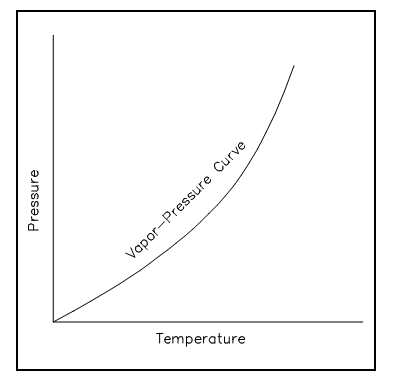
Figure: Vapor Pressure Curve