Critical Point
At a pressure of 3206.2 psia, symbolized by line MNO, there is no constant-temperature vaporization process. Instead of, point N is a point of inflection, with the slope being zero. Such point is termed as the critical point, and at critical point the saturated-liquid and saturated-vapor states are the same. The pressure, temperature, and specific volume at the critical point are termed as the critical pressure, critical temperature, & critical volume.
The constant pressure process is more than the critical pressure and is denoted by line PQ. There is no definite change in phase from liquid to vapor and no definite point at which there is a change from the liquid to the vapor phase. For pressures more than the critical pressure, the substance is generally termed as a liquid whenever the temperature is less than the critical temperature (i.e., 705.47°F) and a vapor or gas whenever the temperature is more than the critical temperature. In the figure shown below, line NJFB symbolizes the saturated liquid line, and the line NKGC symbolizes the saturated vapor line.
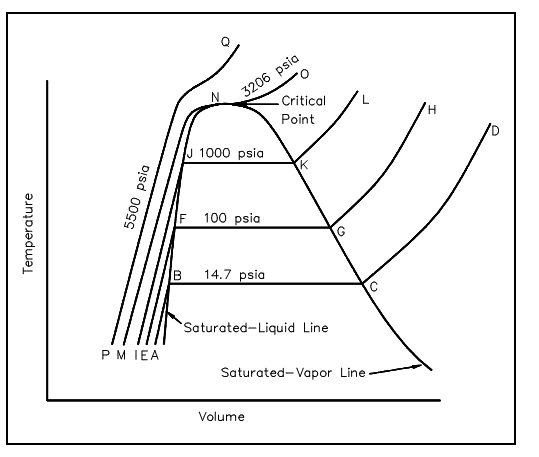
Figure: T-V Diagram