Refractory Metals
The elementary metals that have high melting point, arbitrarily those having melting point above 2450oC are defined as refractory metals. Their alloys also illustrate similar properties. Five such metals from groups VB, VIB and VII B of periodic table are identified. Table explains these metals & some of their physical properties. The refractory metals have very high room temperature ultimate tensile strength but with increasing temperature this strength decreases considerably. For instance, at 1000oC, W has UTS of 250 MPa, Mo and Ta have UTS of 125 MPa. UTS of Nb is still less. Considerable improvement in strength results by way alloying these metals.
Table: Refractory Metals and Their Selected Properties
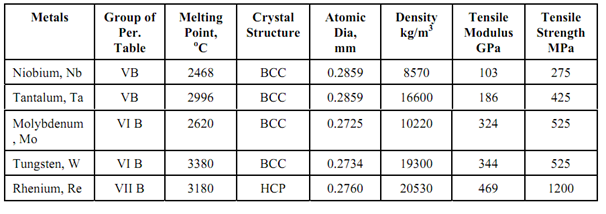
While these refractory metals are recognized with high melting point temperature there is distinction among them. Mo & W have much higher modulus of elasticity than Nb and Ta Nb and Ta have much higher solid solubility for interstitial elements (C, O, H, N) than Mo and W, Nb as well has high superconducting transition temperature of 9.46 K. Thus Nb is useful from very low temperature (near absolute zero) to extremely high temperature.
Figure: Modulus of Elasticity as a Function of Temperature
Mo and W also have significantly higher creep resistance than Nb and Ta, while compared at equivalent fraction of their melting point temperature (T/Tm in K). The higher creep strength of Mo & W is attributed to their higher elastic moduli & low diffusivities. The ductile-to-ductile fracture transition temperature behaviour of recrystallised Nb, Ta, Mo and W is illustrated in Figure. The element Nb & Ta have DBTTS well below room temperature and thus are much easier to fabricated at room temperature than Mo and W, which have DBTTs close to above room temperature. Ta is exceptionally ductile even upto 4 K. Grain size, impurity content & amount of prior cold work have considerable influence on DBTTs.
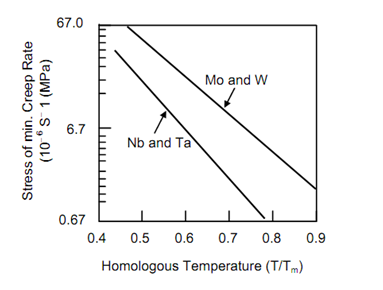
Figure: Creep Strength of Refractory Metals
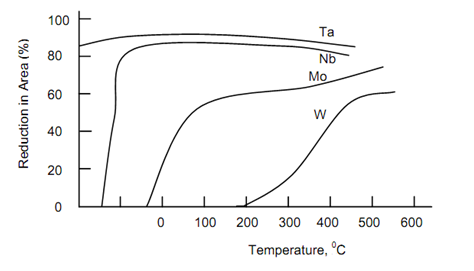
Figure: Ductile to Brittle Fracture Transition