Glasses
Glass is a ceramic material in that it is built from inorganic materials at high temperatures. All commercial glasses are depending on silica to which might be added other oxides which progressively reduce softening point, increase thermal expansion and affect other characteristics. The glasses have been fundamentally identified with requirement of transparency, strength & resistance to corrosion. For this cause it has become indispensable as automobile & house window glazing. These materials have good electrical insulating properties and therefore very widely utilized in electrical and electronic appliances and components.
Glass is achieved as fused mass after cooling to a rigid non-crystalline solid. No long order to repetitive order to molecules exists in glass. Crystallization does not takes place during solidification and therefore cooling curve (a plot among temperature and specific volume) is a continuous curve contrary to cooling curve of a crystalline material which shows a sudden drop in specific volume while solidification begins. Figure compares coming of glass and crystalline solid.
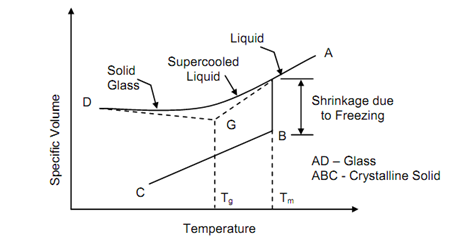
Figure: Solidification of a Crystalline and Glassy Substance
Specific volumes of cooling fused glass & a crystalline melt from a point are plotted as function of temperature. At definite point represented by temperature Tm, the crystalline solid crystallizes causing enhance in density or decrease in specific volume. Crystalline solidification continuous from B to C. Alternatively the glass cools continuously and turns gradually into viscous liquid and viscosity enhances so much that it becomes a solid. It is simply seen that cooling curve of glass contain two distinct region along two slopes. If tangents to these parts are drawn they meet at a point as G. The temperature corresponding to point G illustrated as Tg is called glass transition temperature.
The silica glasses include :
1. Fused silica which contains small bubbles and is utilized for applications that require good thermal shock & corrosion resistance.
2. Fused quartz that is transparent and used for high quality tubing, and
3. Vitreous silica which is utilized for high quality optical components. Aluminosilicate glasses might have service temperature next to silica glass. These glasses are resistant to alkalis. They are utilized for high performance electronic applications and other industrial applications. Borosilicate glass softens at slightly lower temperature than aluminosilicate glasses. As they cost less, they discover wider application. Sodalime glasses have till lower softening temperature & higher coefficient of thermal expansion. They are yet cheaper and are utilized as window glasses in buildings.
Alkali-lead silicate glasses have refractive indices & dispersive power needed for optical instruments. These glasses contain lowest softening temperature.
Tempered glass is strengthened by rapid air cooling or surfaces from softening temperature. This results in residual compressive stresses on the surface & tension inside. The whole strength, particularly impact strength of this glass is developed. Immersing sodium glass in potassium nitrate causes sodium atoms (smaller) replaced by potassium atoms (larger). On surface the residual compressive stress is thus introduced. Tempered glass is used in automobiles.
There are several special purpose glasses in use. They include semi-conducting, photochromic, ophthalmic & sealing glasses. With glass ceramics there exists a great convenience that properties might be tailor made according to requirements. Following properties might be controlled as per requirement.
1. Thermal expansion might be matched with that of the material to which the glass is to be utilized or it might be designed to be zero.
2. Desired refractoriness might be generated.
3. Colour of glass may be incorporated as per desire.
4. Glass might be made machinable.