Properties:
Carboxylic acids and their derivatives are polar molecules because of the polar carbonyl group and the existence of a heteroatom in the group Y. Carboxylic acids can related with each other as dimers through the creation of two intermolecular hydrogen bonds that means that carboxylic acids comprises higher boiling points as compared to alcohols of comparable molecular weight. It means that low molecular weight carboxylic acids are soluble in water. Though, as the molecular weight of the carboxylic acid rises, the hydrophobic character of the alkyl portion eventually outweighs the polar character of the functional group like that higher molecular weight carboxylic acids are insoluble in water.
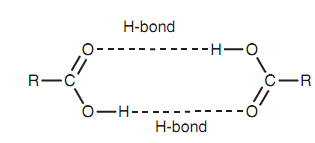
Figure: Intermolecular H-bonding.
Primary amides and secondary amides as well have a hydrogen able of hydrogen bonding (that is RCONHR' , RCONH2), resultant in higher boiling points for these compounds as compared to aldehydes and ketones of identical molecular weight. Acid chlorides, acid anhydrides, esters, and tertiary amides are polar, but lack a hydrogen atom able of taking part in hydrogen bonding. The result of it is that they have lower boiling points than carboxylic acids or alcohols of identical molecular weight, and identical boiling points to comparable aldehydes and ketones.
Carboxylic acids are much weak acids in aqueous solution, creating equilibrium among the free acid and the carboxylate ion. In the existence of a base like sodium hydroxide or sodium hydrogen carbonate, they ionize to make water-soluble salts and this gives a method of separating carboxylic acids from other organic compounds.