Preparations of Carboxylic Acid Derivatives:
Acid chlorides
Acid chlorides can be ready from carboxylic acids by using thionyl chloride (SOCl2), phosphorus trichloride (PCl3), or oxalyl chloride (ClCOCOCl). The mechanism for these reactions is quite involved, but generally includes the OH group of the carboxylic acid working like a nucleophile to make a bond to the reagent and displacing a chloride ion. This has three significant consequences. Very first, the chloride ion can attack the carbonyl group to introduce the needed chlorine atom. After that, the acidic proton is no longer present and so an acid-base reaction is prohibited.
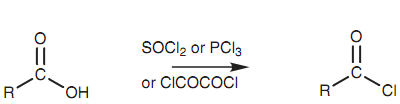
Figure: Preparation of acid chlorides