Oxygen Compounds
Even though carbon creates the molecular oxides CO and CO2 with several bonding, stable oxides of Si and Ge are polymeric. Silica SiO2 has several structural forms that are based on networks of corner-sharing SiO4 tetrahedra. GeO2 can develop in silica-like structures with the rutile structure with six-coordinate Ge. For SiO2 this structure is stable simply at very high pressures, the variation being attributable to the greater size of Ge. Thermodynamically unstable solids GeO and SiO can be made but willingly disproportionate to the ioxide. CO2 is quite soluble in water but true carbonic acid is exist in only low concentration:
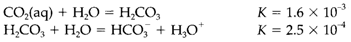
The noticeable Ka given through the result of these two equilibria is 4.5×10-7 (pKa= 6.3), much smaller than the true value for carbonic acid, that is almost in accordance with Pauling's rules (pKa=3.6). The hydration of CO2 and the opposite reaction are very slow, and within biological systems are catalyzed through the zinc-containing enzyme carbonic anhydrase.
SiO2 and particularly GeO2 are less soluble in water than is CO2, even though solubility of SiO2 rises at high pressures and temperatures. Silicic acid is a complex mixture of polymeric forms and only under extremely dilute circumstances is the monomer Si(OH)4 formed. SiO2 reacts with aqueous HF to give [SiF6]2-. The carbonates' structural chemistry, silicates and germanates depicts parallels with the dissimilar oxide structures. All carbonates (example CaCO3) have distinct planar CO32- anions. Silicate structures are based on tetrahedral SiO4 groups that can be isolated units like in Mg2SiO4, but frequently polymerize by Si-O-Si corner-sharing links to give rings, sheets, chains, and 3D frameworks. Several germanates are structurally idnetical to silicates, but germanium more willingly adopts six-coordinate structures.