Other Compounds
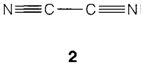
Carbon disulfide CS2 has related bonding to CO2, but SiS2 be different from silica in having a chain structure that is based on edge-sharing tetrahedra and GeS2 accepts the CdI2 layer structure with octahedral Ge. Nitrogen compounds involve the toxic species cyanogen (CN)2 (2) and the cyanide ion CN-, that forms strong complexes with several transition metals. Si2N2O and Si3N4 are polymeric compounds with single Si-N bonds, both forming refractory, chemically and hard resistant solids of interest in engineering applications.
Compounds with metals depict a great diversity. Some silicides and carbides of electropositive metals, like Al3C4 and Ca2Si, could be formulated along with C4- and Si4- ions even though the bonding is surely not very ionic. Compounds through transition metals are metallic in character, those of Si and Ge being usually considered as intermetallic compounds, those of carbon like interstitial compounds with small carbon atoms taking place holes in the metal lattice. Some like TaC and WC are extremely hard, high melting and chemically unreactive, and are employed in cutting tools. Fe3C take place in steel and contributes to the mechanical hardness. Several compounds with E-E bonding are well-known. CaC2 has C2 2- ions (isoelectronic with N2) and responds with water to give ethyne C2H2. Alternatively, KSi and CaSi2 are Zintl compounds with single-bonded structures. Ge (like Sn and Pb) creates some polyanions like [Ge9]4- . Organometallic compounds consisting of metal-carbon bonds are created through almost all metals, and are discussed under the appropriate elements. Some similar Si and Ge compounds are well-known.