Hydrides and organic compounds
Compounds of carbon with hydrogen and other elements form the huge area of organic chemistry. germanes and Silanes are Ge and Si analogs of methane and short-chain saturated hydrocarbons, and can be prepared through various methods, like reduction of halides with LiAlH4:
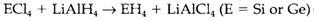
They are much more reactive than corresponding carbon compounds and will inflame impulsively in air. Stability reduces with chain length in series such as
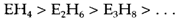
Several derivatives can be made in which H is replaced by monofunctional groups like halide, alkyl, -NH2. Several Si and Ge compounds are identical in structure to those of carbon, but the trisilylamine (SiH3)3N and its germanium analog vary from (CH3)3N in being nonbasic and having a geometry which is planar than pyramidal about N. This proposes the involvement of the N lone-pair electrons within partial multiple bonding by the valence expansion of Si or Ge.
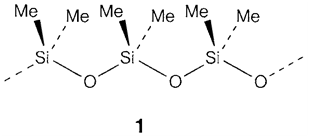
Si and Ge analogs of compounds in which carbon forms double bonds are very much harder to make. (CH3)2SiO is not same propanone (CH3)3C=O, but create silicone polymers with rings or chains that are having single Si-O bonds (1). Attempts to create alkene analogs R2Si=SiR2 (in which R is an organic group) usually result in single-bonded oligomers, apart from very bulky R- groups like mesityl (2,4,6(CH3)3C6H2-), that prevent polymerization.