Hyper conjugation
Both carbons of an alkene are sp2 hybridized. Though, this is altered on creation of the carbocation as shown in figure. While an alkene reacts with an electrophile like a proton, both electrons in the π bond are employed to create a new σ bond to the electrophile.
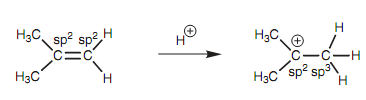
Figure: Hybridization of alkene and carbocation.
As a result, the carbon that gains the electrophile becomes a sp3 center. Another carbon consisting of the positive charge remains like a sp2 center. The meaning of this is that it has three sp2 hybridized orbitals (employed for the three σ bonds still present) and one vacant 2p orbital that is not take part in bonding. Hyper conjugation includes the overlap of the vacant 2p orbital with a neighboring C-H σ-bond orbital.
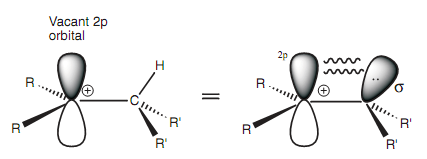
Figure: Hyper conjugation.