Brønsted-Lowry acids:
A Brønsted-Lowry acid is a molecule that consists of acidic hydrogen. In order to be acidic, the hydrogen has to be slightly positive or electrophilic. This is probable if hydrogen is attached to an electronegative atom like a halogen, oxygen, or nitrogen. The subsequent mineral acids and functional groups consist of hydrogens that are potentially acidic shown in the below diagram.
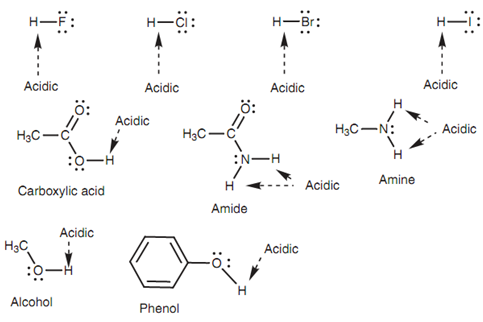
Figure: Acidic protons in mineral acids and common functional groups.
Hydrogens connected to carbon are not generally acidic. Though, we shall look at special cases in which hydrogens connected to carbon are acidic.