Brønsted-Lowry bases:
A Brønsted-Lowry base is a molecule that can make a bond to a proton. Instances include negatively charged ions along with a lone pair of electrons shown in the below diagram.
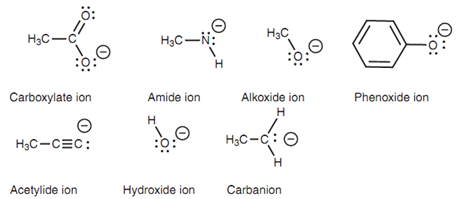
Figure: Examples of Brønsted-Lowry bases.
Neutral molecules can as well work as bases if they consist of an oxygen or nitrogen atom. The most general instances are amines. Though, water, ethers and alcohols are also able of acting like bases as shown in the diagram.
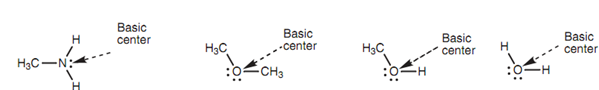
Figure: Examples of neutral Brønsted-Lowry bases.