Trends in pK values
A comprehensive thermodynamic analysis of protolysis needs a cycle that involoves the solvation of both A- and HA. Entropy is significant due to the ordering of water molecules about small ions and species with strongly localized charges . So, the entropy changes will tend to decrease the acid strength of every species giving a conjugate base with strongly localized negative charge. For positive ions protolysis decreses the charge and entropy contributions will raise the acid strength. Even though solvation influences make a rigorous analysis hard, some simple trends can be observed.
- AHn compounds: acid strength raises from left to right in the periodic table, for instance,
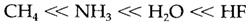
This trend is just associated to the electronegativity increase of the element which is attached to hydrogen that gives more bond polarity in the direction Aδ--Hδ+. Acid strength also rises down the group, for instance,
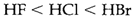
that is not the order supposed from electronegativity. Changes of solvation are significant, but to the trend one simple contribution is the get decreasing H-X bond strength down the group.
The oxides of nonmetallic elements are usually acidic and provide oxoacids in water (example HNO3 and H2SO4). Hydroxides and Oxides of metals tend to be basic and form aqua cations. Though, metals in high oxidation states can also create oxoacids.
- The strengths of oxoacids can be supposed approximately by Pauling's rules.
(i) Writing the formula like XOp(OH)q the first pKa relies largely on the value of p, being approximately equal to 8-5p irrespective of q. Instances with their pKa values are: p=0 (HOCl, 7.2), p=1 (H3PO4, 2.1), p=2 (H2SO4, -2) and p=3 (HClO4, -10).
(ii) PKa increases by almost five units for each subsequent protolysis step, for polyprotic acids (example H2PO4-7.4; HPO42- 12.7).
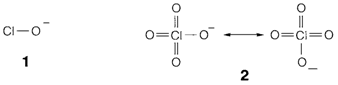
Even though solvation plays a vital role in these trends, the simplest description of rule (i) is that larger values of p provide more scope for the negative charge to be delocalized over the anion. For an instance, in ClO- (1) the formal charge is confined to one oxygen atom, where in CIO4- (2; just two of the four equivalent resonance structures are displayed) it is spread evenly over four.
- Aqua cations: several metal cations are acidic in water. Table 1 depicts that aqueous Fe3+ is a stronger acid than HF.
Acidity may be related with the 'polarizing' power of a cation related with deviations from the ionic model. Strongly acidic cations consists either a high charge/size ratio (example. Be2+, Al3+, Fe3+) or are arrive from metals with low electropositive character (example Hg2+). Salts consisting of these ions form rather acidic solutions, and if the pH is get increased successive protolysis might lead to precipitation and polymerization of an insoluble oxide or hydroxide like Al(OH)3 Some of these compounds depict amphoteric behavior and dissolve in alkaline solution to provide oxoanions. So Al(OH)3, that is very insoluble in a neutral pH range, dissolves at pH>10 to form [Al(OH)4]-.