Strong and weak behavior
The equilibrium constant of the protolysis reaction
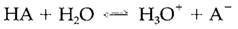
is known as the acidity constant or the acid dissociation constant (Ka) of HA:
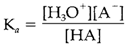
It is frequently expressed as a pKa value, described as
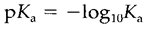
(Note: a larger Ka value that corresponds to a smaller pKa.) A selection of Ka and pKa values is provided in Table 1. If pKa<0 (that is Ka>1) the equilibrium lies strongly to the right and HA is termed as strong acid. Acids with pKa>0 (i.e. Ka<1) are
Table 1. Some acidity constants in water at 25°C
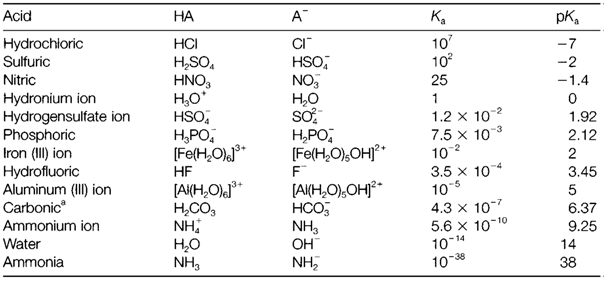
weak acids and go through only partial protolysis. Strong acids in water involve HCl and H2SO4, where HF and HSO4- are weak acids.
In a identical way it is possible to describe the basicity constant Kb and the subsequent pKb from the equilibrium We can differentiate strong bases with pKb<0 and the equilibrium lying to the right side (instances being O2- and NH-2 ) and weak bases with pKb>0 (example NH3 and F-, with pKb equivalent to 4.75 and 10.55, correspondingly). Though, the make use of pKb is not necessary, like the base reaction above might be merged with autoprotolysis to depict that in which pKa refers to the conjugate acid BH+. So the pKa values in Table 1 can be employed to measure the pKb values for the conjugate bases A-.
As a strong acid like HCl is fully protolyzed it is not possible to study this species itself in water. H3O+ is efficiently the strongest acid feasible there and any stronger acid is said to be leveled. In the same way, strong bases like NH-2 are leveled to the strongest base feasible in water, OH-. Solvent leveling limits the range of acid-base nature which can be observed in a given solvent and is one reason for using another solvents with distinct leveling ranges. For instance, liquid ammonia is very basic that is compared with water and H2SO4 is very acidic.