Oxygen compounds
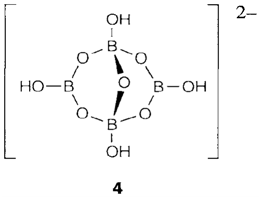
Boric oxide B2O3 is extremely hard to crystallize; the glass have a linked covalent network where both bridging B-O-B and terminal B=O bonds may be exist. The hydroxide boric acid B(OH)3 is formed through the hydrolysis of several boron compounds. It has a layer structure creates of planar molecules linked through hydrogen bonding. It is a Lewis acid that acts like a Brønsted acid in protic solvents. In water the equilibrium
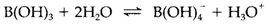
provides a pKa=9.25 but complexing can increase the acidity; for instance, in anhydrous H2SO4 it creates [B(HSO4)4]- and is one of some species that can act like a strong acid in that solvent. Borates can be created with all metals, even though those of groups 1 and 2 are best known. The structural characteristics are rival and complex those of silicates. Boron can take place as planar BO3 or tetrahedral BO4 groups, frequently linked through B-O-B bonds as in silicates. For instance, 4 depicts the ion found in borax Na2[B4O5(OH)4].8H2O, in which both three- and four-coordinate boron is exist. Borosilicate glasses (like 'Pyrex') have lower coefficients of thermal expansion than pure silicate glasses and that's why are more resistant to thermal shock.