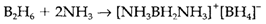Hydrides
The most simple hydrogen compounds are salts of the tetrahydroborate ion BH4- that is isoelectronic and tetrahedral with methane. LiBH4 is ready by reducing BF3 with LiH. It is more extensively used as the sodium salt, that is a powerful reducing agent with enough kinetic stability to be employed in aqueous solution. Reaction of NaBH4 with either I2 or BF3 in diglyme (CH3OCH2)2O provides diborane B2H6, the most simple molecular hydride. Its structure along with bridging hydrogen atoms needs three-center two-electron bonds:
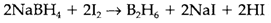
Heating B2H6 above 100°C leads to pyrolysis and makes several more complex boranes of that tetraborane (10) B4H10 and decaborane(14) B10H14 are the most stable. Another reactions can lead to anionic species, like the icosahedral odecahydrododecaborate(2-) [B12H12]2-, get ready at 180°C:
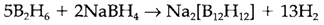
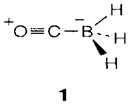
The structural classification and bonding in boranes is explained in Topic C7; particularly striking are the anions [BnHn]2- with closed polyhedral structures. Boranes with heteroatoms can also be prepared, like B10C2H12, that is isoelectronic with [B12H12]2-. Boranes are strong reducing agents and the neutral molecules inflame impulsively in air, even though the anions [BnHn]2- have significant kinetic stability. Diborane itself responds with Lewis bases. The most simple results can be considered as donor-acceptor complexes with BH3, that is a 'soft' Lewis acid and forms adducts with soft bases like CO (1). More complex results frequently result from unsymmetrical cleavage of B2H6, for instance,