Halides
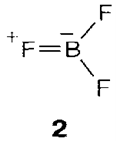
Molecular BX3 compounds are created with all halogens. They contain the trigonal planar structure (D3h) predicted through VSEPR (see Topics C2, C3), even though there show to be a specific degree of π bonding (strongest in BF3) involving halogen lone-pairs and the empty boron 2p orbital (see 2 for one of the possible forms of resonance). The halides are strong Lewis acids, BF3 and BCl3 being employed as catalysts (example in organic Friedel-Crafts acylations). Interaction with a donor gives a tetrahedral geometry approximately boron as along with the analogous BH3 complex 1. The π bonding in the parent molecule is lost and for this cause BF3, where such type of bonding is strongest, is more resistant to adopting the tetrahedral geometry than are the heavier halides. So the acceptor strengths follow the order
BF3 < BCl3< BBr3< Bl3
that is the opposite of that found with halides of most other elements
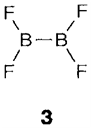
Strongest interaction take place with hard donors like F- (forming the stable tetrafluoroborate ion [BF4]-) and with oxygen donors like water. Apart from with BF (in which the B-F bonds are very strong) complex formation frequently leads to solvolysis, forming B(OH)3 in water. BF3 itself creates a 1:2 aduct with water, that in the solid state can be formulated like [BF3(H2O)].H2O, one water molecule being coordinated to boron through an oxygen lone pair and the other held independently through hydrogen bonding. On melting at 6°C an ionic liquid consisting of [H3O]+ and [BF3(OH)]- is obtained. Pyrolysis of BX3 compounds leads to halides with B-B bonds, for instance, B2X4 (3 with X=F or Cl) and polyhedral BnCln molecules (n=4, 8, 9).