Hybridized centers:
All the atoms in an organic structure (apart from hydrogen) are sp, sp2 or sp3 hybridized.
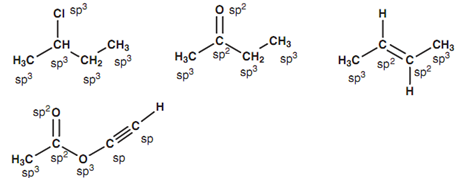
Figure: Examples of sp, sp2 and sp3 hybridized centers.
The recognition of sp, sp2 and sp3 centers is very simple if you keep in mind the following rules:
- All atoms connected by a single bond are sp3 hybridized (apart from the hydrogen).
- Both carbon atoms included in the double bond of an alkene (C=C) have to be sp2 hybridized.*
- Both the carbon and the oxygen of a carbonyl group (C =O) have to be sp2 hybridized.
- All aromatic carbons have to be sp2 hybridized.
- Both atoms included in a triple bond have to be sp hybridized.
- Hydrogen employs a 1s orbital for bonding and is not hybridized.
Hydrogen atoms cannot be hybridized. They can just only bond through using an s orbital because there are no p orbitals in the first electron shell. Hence it is not possible for hydrogen to be included in π bonding. Alternatively oxygen, nitrogen and halogens can form hybridized orbitals that are either included in bonding or in holding lone pairs of electrons.